

1. Stable metal-organic frameworks (MOFs): design, synthesis and modification.
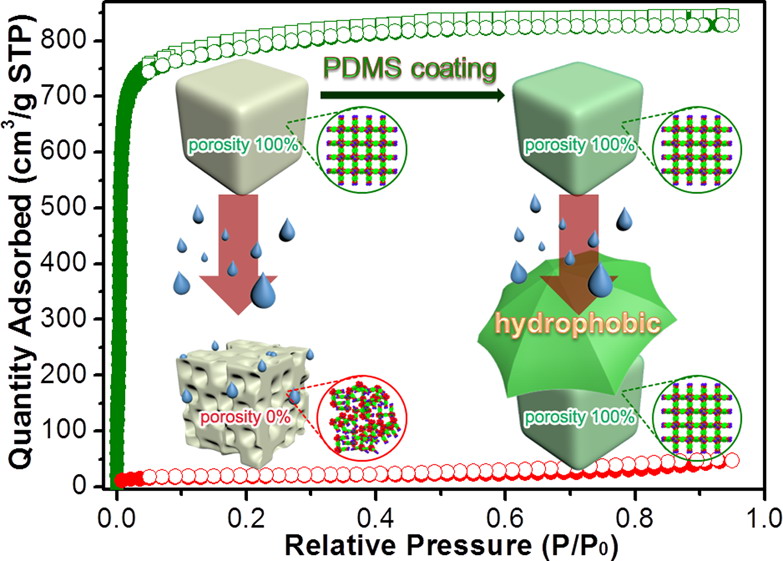
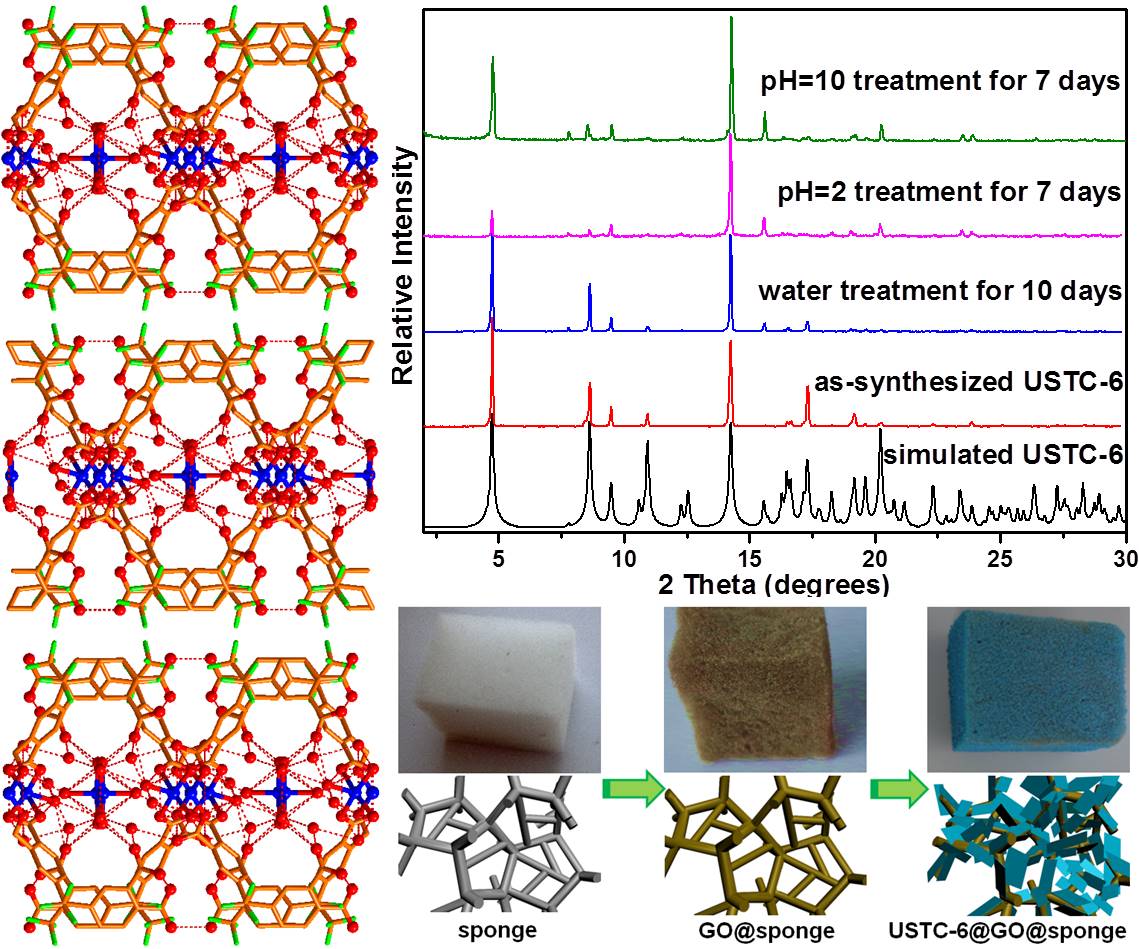
In recent two decades, metal-organic frameworks (MOFs), also known as porous coordination polymers, have received tremendous attention not only because of their powerful attributes on structural and chemical versatility and tailorability, but also due to their potential applications in a variety of domains. Despite this, stability has being recognized as a crucial issue on the way to practical applications of MOFs. As porous functional materials, their thermal stability (generally, higher than 250 degC) is acceptable. However, most MOFs are more or less sensitive to moisture, which could be one of the key limitations to meet the requirements of various applications.
We are keenly interested in the design and synthesis of MOFs that are stable under moisture or water conditions, or even chemically resistant in an acidic or basic medium, based on different synthetic approaches. Alternatively, we try to find effective strategies to post-modify MOFs to make them more stable. These would definitely endow MOFs broader functionalities and applications in a real world.
Representative articles:
J. Am. Chem. Soc. 2017, 139(27), 9136-9139.
J. Am. Chem. Soc. 2016, 138(16), 5316-5320.
J. Am. Chem. Soc. 2014, 136(49), 16978-16981.
ACS Catal. 2018, 8(5), 4583-4590.
NPG Asia Mater. 2016, 8, e253.
Chem. Commun. 2016, 52(33), 5734-5737.
2. MOFs and their composites/derivatives: fabrication and catalytic applications
a) MOFs for catalysis
The adjustable metal centers and funtionalizable organic linkers as well as modifiable pore walls provide multiple opportunities to create desirable, high-density and uniform active sites for catalysis. Meanwhile, the high porosity greatly facilitates the mass transportation of substrates/products. Therefore, MOFs possesses the merits from the common homogeneous (readily accessed active sites) and heterogeneous (recyclability) catalysts, and are thus very suitable for catalysis, including heterogeneous catalysis in organic reactions, photocatalysis and even electrocatalysis.
In addition, compared to the traditional porous materials, like zeolites and mesoporous silica, MOFs have their own advantages for catalytic applications. Although zeolites have been regarded as one of the most commercially important catalysts, the small pore size of zeolites is usually a key limitation to address the catalytic reactions with large substrates; while mesoporous silicate materials, such as the MCM- and SBA-series of materials, have pores that are too large which don¡¯t have confinement and selectivity effects for catalytic substrates. Porous MOFs have an abundant diversity of structures and pore sizes ranging from non-pores to mesopores. Therefore, MOFs are regarded as bridging the gap between zeolites and mesoporous silica and exhibit size-selective catalysis in various reactions.
We have elaborately selected stable MOFs with high-density of active sites for particular reactions. Excellent catalytic activity, recyclability and even selectivity have been obtained based on MOF catalysts.


Representative articles:
Acc. Chem. Res. 2019, 52(2), 356-366.
Mater. Today 2019, 27, 43-68.
EnergyChem 2019, 1(1), 100005.
Chem. Soc. Rev. 2019, 48(10), 2783-2828.
J. Am. Chem. Soc. 2015, 137(42), 13440-13443.
Angew. Chem. Int. Ed. 2017, 56(2), 563-567.
ACS Cent. Sci. 2018, 4(1), 105-111.
Adv. Mater. 2018, 30(37), 1703663.
Chem. Sci. 2018, 9(12), 3152-3158.
ACS Catal. 2016, 6(8), 5359-5365.
Chem. Mater. 2016, 28(18), 6698-6704.
ChemSusChem 2015, 8(5), 878-885.
Chem. Eur. J. 2014, 20, 14976-14980.
Chem. Commun. 2014, 50(9), 1092-1094.
b) MOF-based nanocomposites for catalysis
The size-tunable cages or channels in MOFs allow them to host or support metal nanoparticles (NPs) for catalysis over a broader scope; the crystalline porous structure of MOFs is expected to limit the migration and aggregation of metal NPs, which is highly desirable in catalysis. In this aspect, Jiang has a series of early-stage reports (JACS 2009, 131, 11302; JACS 2011, 133, 1304; JACS 2011, 133, 11822; Chem. Commun. 2011, 47, 3351; Chem. Eur. J. 2011, 17, 78; etc.) during his postdoc period working in Japan (with Prof. Qiang Xu).
Most of the previous work about the catalytic functions of metal NPs@MOF was based on metal NPs as the only active site. The catalysis by judiciously synergizing the respective advantages from each component in the composites (involving MOFs and metal NPs) will be the future targets, just for examples, the combination of host and guest cooperative catalysis, the one-pot cascade catalysis, or the combination of heterogeneous catalysis and photocatalysis, etc.


Representative articles:
Chem 2019, 5(4), 786-804.
Chem. Soc. Rev. 2017, 46(15), 4774-4808.
J. Am. Chem. Soc. 2017, 139(5), 2035-2044.
Angew. Chem. Int. Ed. 2016, 55(11), 3685-3689.
Angew. Chem. Int. Ed. 2016, 55(26), 7379-7383.
Angew. Chem. Int. Ed. 2016, 55(32), 9389-9393.
Angew. Chem. Int. Ed. 2018, 57(4), 1103-1107.
Angew. Chem. Int. Ed. 2018, 57(19), 5379-5383.
Angew. Chem. Int. Ed. 2019, 58(31), 10713-10717.
Angew. Chem. Int. Ed. 2019, 58(35), 12175-12179.
Adv. Mater. 2018, 30(7), 1705112.
Adv. Mater. 2018, 30(27), 1707377.
Nat. Commun. 2019, 10, 3462(1-10).
ACS Catal. 2015, 5(4), 2062-2069.
Mater. Horiz. 2015, 2(6), 606-612.
Small 2015, 11(1), 71-76.
On the other hand, given that metal clusters and organic linkers are well organized in the framework, MOFs with superb structural tunability offer congenital conditions as precursors or templates for constructing nanocomposites or porous carbons, primarily via pyrolysis. In the solid-solid transformation process, MOFs behave as ideal sacrificial templates and their porosity can be partially preserved. Although in its infancy, it is highly possible and expected that, upon thermal decomposition of MOF hard templates, the resultant nanocomposites or porous carbons with preserved MOF morphology, could be well accessible and very effective for heterogeneous catalysis and electrocatalysis.



Representative articles:
Coord. Chem. Rev. 2018, 362, 1-23.
Adv. Mater. 2015, 27(34), 5010-5016.
Chem 2017, 2(6), 791-802.
Angew. Chem. Int. Ed. 2018, 57(28), 8525-8529.
Angew. Chem. Int. Ed. 2019, 58(11), 3511-3515.
Chem. Sci. 2016, 7(3), 1690-1695.
Green Chem. 2016, 18(5), 1212-1217.
Chem. Commun. 2015, 51(39), 8292-8295.
Chem. Commun. 2016, 52(22), 4199-4202.
Chem. Eur. J. 2016, 22(10), 3470-3477.